Repetitive Transcranial Magnetic Stimulation
Repetitive Transcranial Magnetic Stimulation (rTMS) is a treatment for depression and possibly other psychiatric disorders. Studies have shown that rTMS is an effective treatment for patients with depression. The following will provide further detailed information about rTMS and its use in the treatment of depression.
About Repetitive Transcranial Magnetic Stimulation (rTMS)
What is rTMS?
rTMS is a procedure that involves the focused application of magnetic energy to superficial regions of the brain, thus inducing small electric currents. During the rTMS procedure, an electrical current passes through a small coil placed close to the scalp. This current induces a magnetic field. The magnetic field can pass into the brain without resistance. If the magnetic field is of sufficient strength, it will stimulate electrical activity in the nerves below the coil, this is, in superficial regions of the brain. This stimulation may be repeated many times per second and with variation and intensity: these variations will determine the effects of the stimulation- rTMS and can be applied in different ways to either increase or decrease local brain activity.
rTMS in Depression
Studies have evaluated the role of rTMS in the treatment of depression since the mid-1990s. These studies have clearly shown that rTMS is more effective than placebo type of stimulation, especially in patients who have not responded well to antidepressant medication treatment. It is not clear how rTMS works in depression, however one theory is that the repeated stimulation alters the sensitivity of nerve cells in the front regions of the brain and alters how active these areas are. When someone receives rTMS under a treatment protocol, it is usually applied for between 20 and 45 minutes, on a daily basis over a course of several weeks.
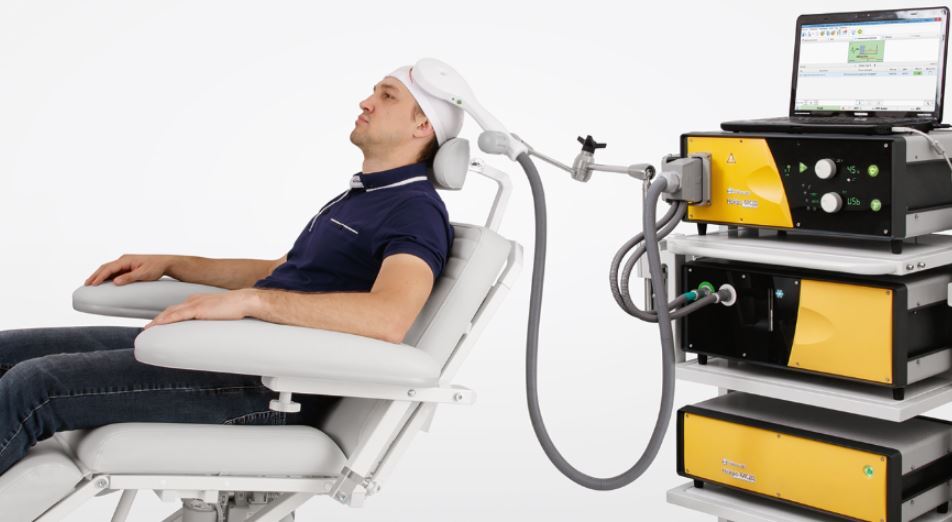
What happens when you have rTMS?
rTMS is usually administered five (5) days per week (Monday to Friday). Scheduled in consultation with your psychiatrist. Sessions usually take between 20 and 45 minutes per day depending on the protocol being utilised. During a rTMS session a patient is awake, alert and aware of what is happening at all times.
Before the treatment course begins some time will be spent stimulating the area of the brain that controls muscle movement in the hand opposite to the side of the brain on which rTMS will be given. The patient may feel small twitches in the hand during this procedure. It is not painful. This procedure is done to establish how high the machine intensity needs to be sent to affect the brain in an individual patient.
During the treatment itself, a coil usually placed on the scalp (held by hand or with a coil stand) near the front region of the brain. This may be on the left or right depending on how the treatment is being given. the coil is connected to a machine that generates the electrical current.
The current produces no sensation as it does not come into contact with the body. as the magnetic field is produced by the electrical current being switched on and off, the machine produces a clicking sound although the patient may wear disposable ear plugs to prevent the discomfort of sound. The patient may feel a tapping sensation under the coil (this occurs due to a twitch produced in scalp muscles as the magnetic field crosses into the brain). The magnetic field can also stimulate small nerves around the head and face, producing a muscle twitch in the forehead, face or eye region. The stimulation can be applied in a variety of ways: the most common two ways are either as a long train of pulses administered over a few seconds at a time.
If the rTMS is effective in treating depression, this usually takes several weeks. Most patients who respond feel different during the second or third week, but they may not feel much better until they have had three (3) or four (4) weeks of treatment.
rTMS is usually given in a defined course to try and achieve a remission or substantial reduction in depression severity. Depression is a relapsing disorder and so it is likely that it will return after treatment for most patients sometime in the future. Therefore, most patients are encouraged to continue with other treatment including antidepressant medications, especially to try and prevent relapse after the treatment with rTMS finishes.
Side Effects
There are several potential side effects that might be experienced during an rTMS procedure. First, a headache or neck-ache can occur, similar to a tension headache, caused by the stimulation of nerves in the scalp. This occurs in approximately five of every one hundred (100) participants studied and will often improve rapidly with simple pain medication such as Panadol. Second, the stimulation may be uncomfortable. As the magnetic field passes into the brain, it can cause stimulation of the muscles in the scalp causing them to contract. This can feel like a tapping sensation. How strong this feels varies dramatically between subjects: some feel almost nothing, and others have a stronger sensation. Those who do find it uncomfortable usually find they get used to the sensation over a few days and strength of the stimulation pulse can be lowered until then.
Risks
The main concern associated with rTMS is its potential to cause a fit or seizure. Safety guidelines to limit the dose of rTMS used started in the late 1990's and there has been a reduced risk of occurrence since then. You should always tell you treating team if you change your medication or experience other medical issues during the course of rTMS as medication changes or medical illness could affect the risk of seizure. We will ask you to wear ear plugs during the rTMS treatment as there is a risk of hearing damage from the sound produced by the machine although this has not been shown with people undergoing rTMS. It is your responsibility to fit the ear plugs provided for the duration of the procedure.
There are several reasons why someone cannot have rTMS. These include the diagnosis of epilepsy, an active brain illness such as stroke or anything that may be affected by the magnetic field. This can include metal implants in the head, surgical clips, cardiac pacemakers, implanted medication pumps or electrodes. rTMS may also be avoided if a patient has an unstable medical condition (for example, heart disease) that could be exacerbated if they were to suffer a seizure. If any of these conditions are relevant to you, it is very important that you let us know prior to undertaking it.
Although unlikely, more recently ophthalmological risks have been identified with undergoing rTMS treatment. This may include contraction of extraocular muscles causing repetitive eye movement and secondary retinal trauma; or delayed visual changes in the hours following treatment. Please ensure you advise your treating team if you experience eye pain or discomfort during the course of your treatment. Alcohol and illicit substances should not be taken during the course of treatment.
Other Treatments Whilst Undertaking rTMS
It is important to tell your doctor and our rTMS staff about any treatments or medication you may be taking, including non-prescription medications or herbal remedies and any changes to these during your participation with rTMS. We typically try and avoid significant changes to medication, or starting new medications that affect the brain during a course of rTMS treatment as it is likely to confuse our understanding about what might have produced any therapeutic benefits with this treatment.
More information
For more information on rTMS you can visit below:
How to access rTMS
Patients interested in rTMS will need a referral to a psychiatrist with admission rights to Hirondelle Private Hospital.
You can contact our Admissions Team via our details below or access our online forms:
Phone: 1800 943 003
Email: hdl.admissions@imh.com.au